|
Trends in Molecular Diagnostics Vol. 1/2018 |
|
|
Micro-Dx™ Received CE IVD |
|
|
Routine Culture-Independent Molecular Diagnosis of Pathogens by Micro-Dx™ CE IVD ·
Unique
automated pathogen enrichment and DNA extraction · Broad-range detection and identification Species from more than 200 bacterial and 65 fungal genera ·
Diversity
of samples ·
Flexibility
|
|
|
Latest Application Note |
|
|
Micro-Dx™ CE IVD – Results From Multi-Sites Clinical Evaluations Micro-Dx™ is the new kit for the in-vitro diagnosis of microbial pathogens without the need for cultivation. The kit is used with the SelectNA™plus instrument which automatically processes a variety of routine specimens ending up with human DNA depleted, pure DNA from eubacterial and fungal pathogens. Assaying is performed by broad-range 16S and 18S rRNA genes PCR or Real-Time PCR analysis which provides a positive-negative result after 3.5 to 4 hours. Identification of species by Sanger sequencing analysis is completed within another 3 to 4 hours. Here, the results of multi-sites evaluations by routine laboratories are presented which either used results from other molecular tests or culture as reference systems. In total, 409 samples were analyzed using Micro-Dx™ in 3 laboratories from Germany and in one laboratory each from Denmark, France, Switzerland and UK. Samples comprised of a variety of tissues, fluid biopsies and swabs. The evaluations indicated acceptable diagnostic performance of Micro-Dx™ with mean values of sensitivity of 89%, specificity of 85%, PPV of 76%, NPV of 88%, and concordance of 85%. In 7 of the 9 evaluations, Micro-Dx™ identified pathogens in reference-negative samples. By its automated sample extraction, Micro-Dx™ contributes greatly to the demand for a reduced hands-on workflow in routine molecular pathogen testing.
|
|
|
SelectNA™plus – Unique Platform for Microbial DNA Enrichment and Isolation Key features: · Benchtop robotic platform for human DNA removal and microbial DNA isolation · Validated for body fluids and tissue biopsies · Flexible extraction: 1 to 12 samples · Internal UV decontamination · Kits: Micro-Dx™ CE IVD and MolYsis-SelectNA™plus
|
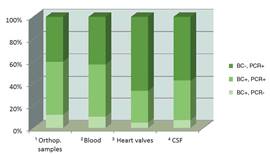
Ratio of positive results by culture (BC), 16S/18S PCR (PCR) or both methods; obtained with orthopaedic samples, blood, heart valves and CSF |
|
Dear reader, Welcome to this edition of Trends in Molecular Diagnostics. With this newsletter we want to keep you updated on the latest developments in molecular detection of microbial infections. Best regards, Michael Lorenz, Ph. D. (Editor in Chief) |
|
|
This information is brought to you by Molzym, Mary-Astell-Str. 10, D-28359 Bremen, Germany.// If this information is not of interest to you and you do not want to receive any scientific & service updates, please disregard and we apologize for any inconvenience this may have caused. To unsubscribe from the list, simply reply with UNSUBSCRIBE in capital letters in the subject line. |
|